Boyle's Law Data Table
Observed 1 Gauge Pressure Ptorr x V mL-k torr Multiply the pressure in Volume V Pressure mL ml psi Torr units by the actual V Average value of k Graphing. Graph the pressure psi units on the y-axis and the actual volume mL on the x-axis by hand.
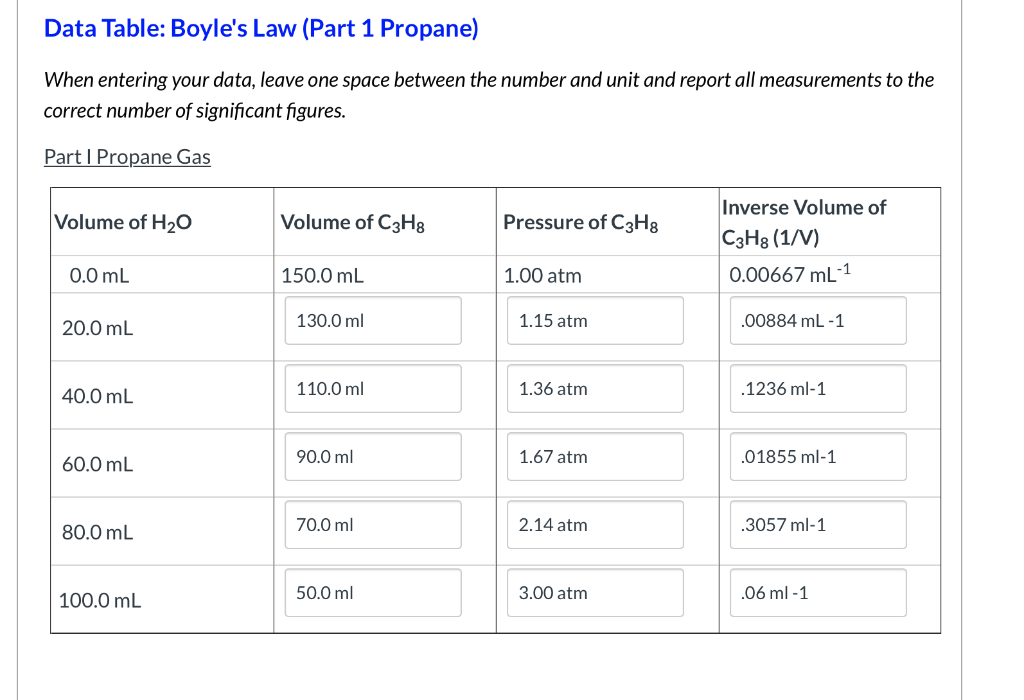
Boyles Law Part 1 Propane When entering your data leave one space between the number and unit and report all measurements to the correct number of significant figures.
. Volume is plotted on the x-axis with the corresponding pressure on the y-axis. The third column of the Data and Calculations table. Is the relationship between pressure and volume direct or inverse.
BOYLES LAW Data table. Using P V and k write an equation representing Boyles law. Volume and Boyles Law Data and Results Table See Post-Lab Question 2.
Boyles law also referred to as the BoyleMariotte law or Mariottes law especially in France is an experimental gas law that describes how the pressure of a gas tends to decrease as the volume of the container increases. Write a verbal statement that correctly expresses Boyles law. Boyles Law Experiment Data Table Volume of gas 05cm3 188 168 148 128 108 88 68 Pressure of gas.
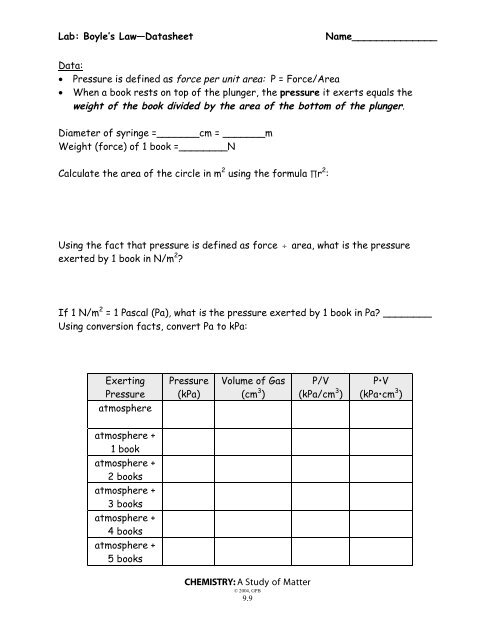
1V graph Attach your P vs. BOYLES LAW EXPERIMENT Dry Lab Pressure Number of books Total pressure atmosphere added 1Total. The constant for each experiment can be different depending on how the experiment is set up.
Record the pairs of data for pressure and propane volume in the table below. Thus pressure is inversely proportional to volume. Boyle Law Question DATA SUMMARY Boyles Law Volume ml Pressure atm 12 096 08 0685714286 06.
Convert the local barometric pressure to psi units and enter the value to the nearest psi in the Data and Results Table. There are several ways to verify the law. Y Ax or.
As observed from the graph above pressure increases with a decrease in volume and vice versa. It is as follows. Record the values in the data table.
Volume of Gas mL Pressure atm 150mL 1 atm 130mL 115 atm. 31st Oct 2019 2 min read. The curve is called PV curve and it is hyperbolic in nature.
Number of Books Mass of Book kg Data Table 1. Good data may show some minor variation but the values for k should be relatively constant. Label the graph properly 2 Repeat graphing the P vs.
Good data may show some minor variation but the values for k should be relatively constant. PV constant so. Microsoft Word - 9-0910 Boyles Law labdoc Author.
A modern statement of Boyles law is. How constant were the values for k you obtained in Question 8. Observing Boyles Law Data Table 1.
Data Sheet for Lab XII 12 Boyles Law Hayden-McNeil Complete the table for propane. Total Volume of Water in mL to Volume of Gas in. However within a single experiment where the temperature and moles of gas remain.
Within bounds of experimental error the relationship between pressure and volume of a gas is PV k PV k. Part I Propane Gas Inverse Volume of Volume of H20 Volume of C3H8 Pressure of C3H8 C3H8 1V 000667 mL-1 100 atm 00 mL 1500 mL 115 atm 1300 ml. Look at the title of this lab Title.
It relates pressure and volume of gas keeping other parameters amount of gas and temperature constant. View Boyles Lawdocx from CHEM 112 at Texas AM University. Boyles Law can be derived from the function used to best fit the curve to the data.
View Notes - Boyles Law from ENGL COMP2 at University of California Los Angeles. See Post-Lab Question 5. The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed syst.
To Verify Boyles Law Experimentally. Volume and Boyles Law continued 4 2016 lnn centc nc. Boyles law is a famous gas law studied in physics and chemistry.
The graph of Boyles law is known as pressure-volume graph or PV curve. The article covers a standard laboratory method to verify the law by studying the relation. Look at the last two columns of your data table.
View Homework Help - Boyles Law Data Table Analysisdocx from ENGLISH 105 at Dublin High School. View Lab Report - Lab 11 Data Tables Boyles Law from CHEM 110 at Butler Community College. For which column PV or PxV were the values relatively constant.
Trinity Mai March 7 2018 Ha 3 Boyles Law Analysis In this lab we tested Boyles law with a. Using P V and k write an equation representing Boyles law. The pressure of a gas decreases as the volume increases making Boyles law an inverse relationship.
Textbook Solutions Expert Tutors Earn. A graph of the data in the table further illustrates the inverse relationship nature of Boyles Law see figure below. P 1 V 1 P 2 V 2.
Boyle Law Question DATA SUMMARY Boyles Law Volume ml Pressure atm 12 096 08 0685714286 06 0533333333 048 Correlation coefficientPvs.

Data Table Boyle S Law Part 1 Propane When Chegg Com

Experiment 12 An Investigation Of Boyle S Law
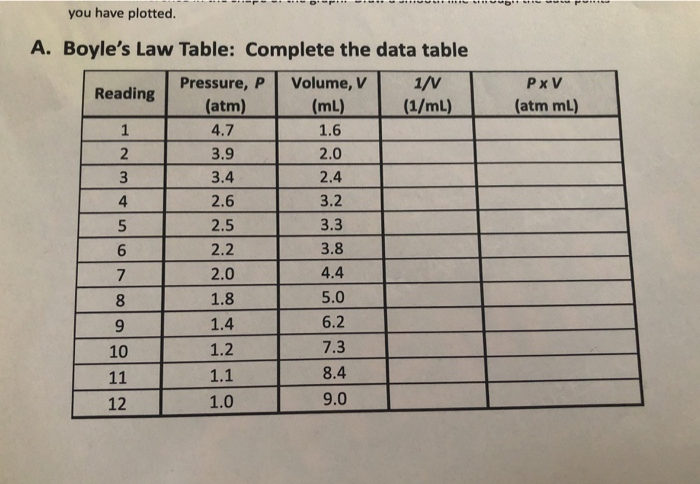
Solved You Have Plotted Pxv Atm Ml 4 A Boyle S Law Chegg Com
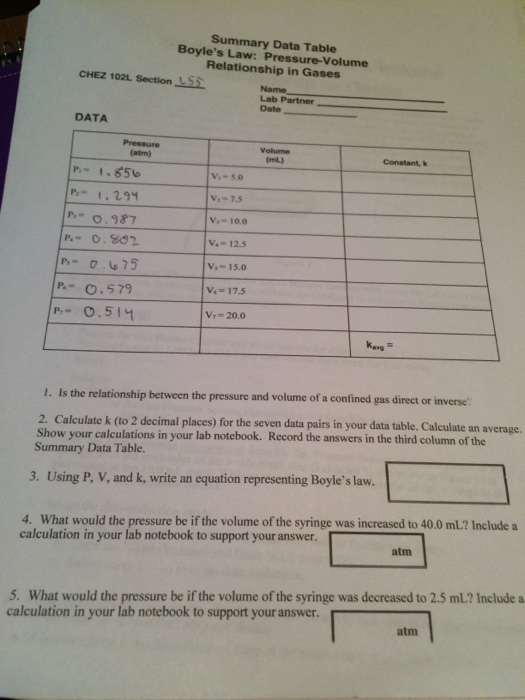
Solved Summary Data Table Boyle S Law Pressure Volume Chegg Com
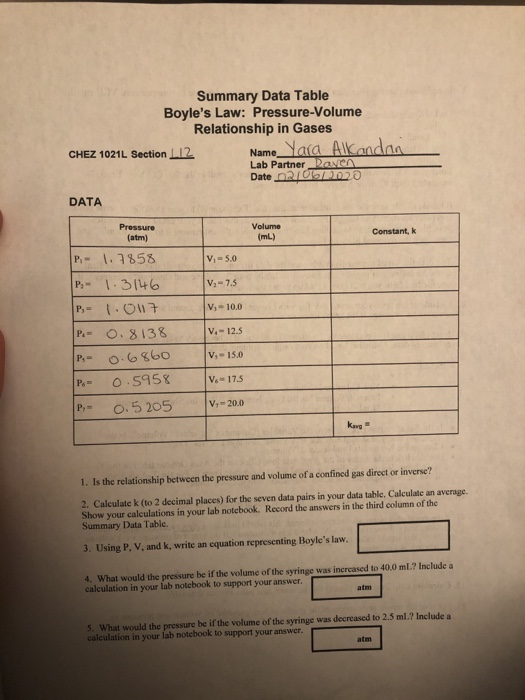
Solved Summary Data Table Boyle S Law Pressure Volume Chegg Com
0 Response to "Boyle's Law Data Table"
Post a Comment